Background
Most patients with primary myelofibrosis (PMF) in pre-/early fibrotic stage (pre-PMF) and at low or intermediate-1 risk by the dynamic international prognostic scoring system (DIPSS) will progress to higher risks or overt MF. There is currently no consensus on the optimal treatment for these patients. Ropeginterferon alfa 2b (ropeg) is a next-generation monopegylated interferon alfa-2b developed specifically to treat myeloproliferative neoplasms (MPN).
Aims
P1101MF is an on-going multicenter phase 2 study of ropeg in patients with pre-PMF and DIPSS low/intermediate-risk PMF.
Methods
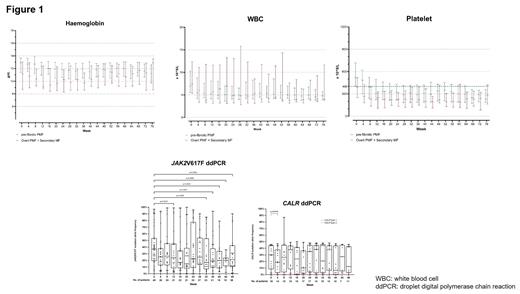
Key eligibility included morphologically confirmed pre-PMF, overt PMF, post-polycythemia vera MF (PPV-MF) or post-essential thrombocythaemia MF (PET-MF), DIPSS low/intermediate-1 risk, and the need for cytoreduction. The primary outcome were responses in haemoglobin (Hb, from 10 g/dL to upper reference range), white blood cell (WBC, < 10 x 10 9/L) and platelet (≤ 400 x 10 9/L) at 24 and 48 weeks. Secondary outcomes included adverse events (AEs), changes in mutant allele frequencies (MAF) of driver and non-driver genes as assessed by droplet digital polymerase chain reaction (ddPCR), quality-of-life (QOL), cytokine profiles and bone marrow morphology. Patients received ropeg at a dose of 250 mcg at week 1, followed by 350 mcg at week 2 and 500 mcg every 2 weeks from week 4 onwards.
Results
At the data cut-off of 30 June 2023, 37 men and 29 women with a median age of 59 (range: 30-86) years were enrolled. The diagnoses were pre-PMF (=46, 69.6%); overt PMF (N=6, 9.1%); PPV-MF (N=5, 7.5%); and PET-MF (N=9, 13.6%). Mutational profile of driver genes was JAK2V617F (N=46, 69.6%); CALR mutations (type 1/type-1 like, N=13, 19.7%; type 2/type 2-like, N=4, 6.1%); MPL mutation (N=1, 1.5%); and triple-negative (N=3, 4.5%). The median time from diagnosis to treatment was 5.8 (1-271) months. The median baseline parameters were white blood cell count (WBC): 6.49 (3.25-33.95) x 10 9/L; haemoglobin (Hb): 12.5 (7.1-15.9) g/dL; platelet count: 495 (113-1114) x 10 9/L; and lactate dehydrogenase: 277 (136-1146) IU/L.
The median follow-up was 69 (4-69) weeks, with 61 patients (92%) and 51 patients (77%) having completed 24 and 48 weeks of treatment respectively. Responses in Hb, WBC and platelet were 75%, 82% and 74% respectively at 24 weeks, and 82%, 80% and 71% respectively at 48 weeks. Reduction of MAF at 24 and 48 weeks was achieved for JAK2V617F in 50% (19/38) of patients (≥50%, N=10, 26%; complete, N=3, 8%) and 79% (22/28) of patients (≥50%, N=6, 21%; complete, N=1, 4%) respectively; and for CALR in 44% (7/16) of patients (≥50%, N=1, 6%) and 50% (5/10) of patients (≥50%, N=1, 10%) respectively. Forty-six patients (69.6%) had bone marrow biopsies serially performed, with 42 patients (91%) having stable/improved marrow fibrosis. Eight patients (17.4%) had resolution of marrow fibrosis by 48 weeks.
There were 5 discontinuations (personal reasons, N=1; intractable pruritus, N=1; symptom/spleen size progression, N=3). Disease progression (pre-PMF to overt PMF; development of accelerated/blast phase) was not observed. Non-haematological AEs included transaminitis (grade 1-2, N=34); malaise (grade 1-2, N=27; grade 3-4, N=1) and hair loss (grade 1-2, N=21). The most common haematological AE was anaemia (grade 1-2, N=15; grade 3-4, N=6).
Conclusion:
Ropeg was well-tolerated, effective in cytoreduction and induced molecular and morphologic responses in patients with pre-PMF and low/intermediate-1-risk MF.
Disclosures
Gill:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Pfizer: Consultancy, Other: Conference support; BMS: Consultancy; GSK: Consultancy; Novartis: Consultancy, Other: Conference Support, Research Funding; PharmaEssentia: Consultancy, Other: Conference Support, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal